Coronavirus is evolving but so are our antibodies
Kateryna Kon/Shutterstock
By Sarah L Caddy, University of Cambridge and Meng Wang, University of Cambridge
The emergence of “variants of concern” has raised questions about our long-term immunity to the coronavirus. Will the antibodies we make after being infected with or vaccinated against the dominant lineage, called D614G, protect us against future viral variants?
To answer this question, scientists have been examining how our antibody responses to the coronavirus develop over time. Several studies have recently compared the difference between antibodies produced straight after a coronavirus infection and those that can be detected six months later. The findings have been both impressive and reassuring.
Although there are fewer coronavirus-specific antibodies detectable in the blood six months after infection, the antibodies that remain have undergone significant changes. Researchers have tested their ability to bind to proteins from the new coronavirus variants and found that 83% of the “mature” antibodies were better at recognising the variants. A recent preprint (a study that is yet to undergo peer review) also found that some antibodies present six months after infection were starting to be able to recognise related, but entirely distinct viruses, such as the coronavirus that causes Sars.
How is this possible? Quite simply because the B cells that make antibodies evolve after they are first activated. While it is well known that viruses can mutate over time, our own B cells can also take advantage of mutations to make superior antibodies.
Somatic hypermutation
A key difference between the mutation of antibodies and viruses is that mutations in antibodies are not entirely random. They are, in fact, directly caused by an enzyme that is only found in B cells, known as Aid (activation-induced deaminase). This enzyme deliberately causes mutations in the DNA responsible for making the part of the antibody that can recognise the virus. This mutation mechanism was solved by pioneering researchers at the MRC Laboratory of Molecular Biology in Cambridge, UK, almost 20 years ago.
AID activity leads to a much higher rate of mutation in B cells than in any other cell in the body. This phenomenon is called “somatic hypermutation”.
Some of the mutations that are induced in the antibody binding site will improve the binding of that antibody to the target virus. But some mutations will have no effect, and others will actually decrease the antibody’s ability to latch onto the target virus. This means there needs to be a system whereby B cells making the best antibodies will be selected.
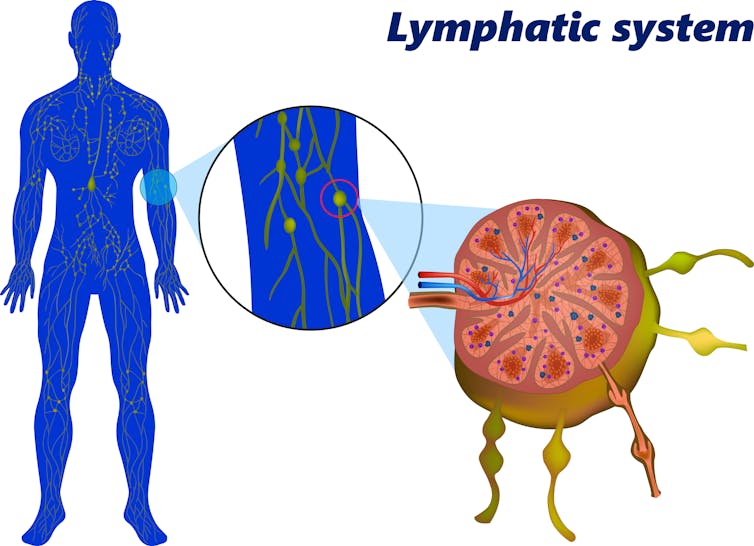
B cells congregate in small glands called lymph nodes while they are developing. Lymph nodes are found all around the body and often get bigger if you are fighting an infection.
Sakurra/Shutterstock
Within the lymph nodes, the B cells that can make better antibodies after somatic hypermutation are given positive signals to make them replicate faster. Other B cells fall by the wayside and die. This “survival-of-the-fittest” process is called affinity maturation; the strength or “affinity” with which antibodies bind to their target matures and improves over time. After this rigorous selection, the newly emerged B cell will now mass produce its improved antibody, leading to a more effective immune response.
The course of a typical COVID infection is ten to 14 days, so the first wave of antibodies driving out the virus doesn’t have long enough to evolve because affinity maturation normally takes place over weeks. But research from the US has shown that small non-infectious bits of SARS-CoV-2 remain in the body after an infection is cleared, so B cells can keep being reminded of what the virus looks like. This allows antibody evolution to continue for months after an infection has been resolved.
Overall, antibody evolution means that if a person is infected with coronavirus for a second time, antibodies with far superior binding ability will be ready and waiting. This has important implications for vaccination. Antibody evolution will begin after the first vaccination so that much-improved antibodies will be present if the virus is encountered at a later date. Hopefully, it is comforting to know that it is not just the virus that is mutating, our own antibodies are keeping pace.![]()
Sarah L Caddy, Clinical Research Fellow in Viral Immunology and Veterinary Surgeon, University of Cambridge and Meng Wang, Cancer Research UK Clinician Scientist Fellow, University of Cambridge
This article is republished from The Conversation under a Creative Commons license. Read the original article.